
BOILING WATER: HOW DO ENVIRONMENTAL CONDITIONS AFFECT THE TEMPERATURE AT WHICH A PURE COMPOUND BOILS?
Date
May 13 - 15
Purpose
Demonstrate the effect of altitude (atmospheric pressure) on the boiling point temperature of water.
Discussion
The air is made of molecules that have a mass and weight. The force of gravity pulls all air molecules toward the planet. This force causes a downward pressure, termed atmospheric pressure. Atmospheric pressure is greatest at an altitude of 0 m. In moving to a higher elevation, there are fewer molecules above pushing down, and therefore the atmospheric pressure decreases. The general statement that follows is, atmospheric pressure decreases with increasing altitude.
Atmospheric pressure pushes down on all objects, including a pot of water. When introducing a heat source such as a burner on a stove or fire energizes water molecules in the pot, they can change state from liquid to gas (e.g. water vapor). The temperature at which a pure compound (e.g. water) is defined to be boiling, is the temperature at which that liquid's vapor pressure above the liquid is equal to atmospheric pressure. This means that a force that prevents a liquid from becoming a gas is the atmospheric pressure, and if the downward force of atmospheric pressure decreases it becomes easier for water molecules to be liberated as gas.
Method
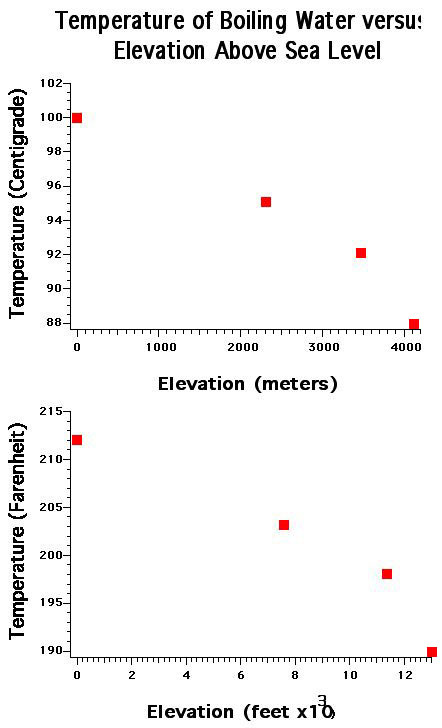
The boiling point of pure water will be measured at several altitudes as the i2P team climbs into the Andes Mountains. Boiling point temperature will graphed against altitude.
School Exercise
Boil water in your classroom and carefully measure the boiling temperature. What is the elevation of your community in meters? Compare your results to those noted on the expedition graph. What does this tell you about the atmospheric pressure where you live?
Experiment Report & Results

Hello all, George reporting in from Uyuni, Bolivia. Prior to leaving my home town in greater Vancouver Canada, I measured the boiling temperature of water at a park near my house at 2 m (6 ft) above sea level. I flew from Vancouver to Toronto on the afternoon of Thursday May 12, ran through the Toronto airport and barely caught the overnight flight to Santiago, Chile. On Friday night, the expedition members were in San Pedro de Atacama, Chile, overnight and I had a chance to make another water boiling temperature measurement. San Pedro de Atacama is at an elevation of 2308 m (7605 ft). The boiling water boiling temperatures that I measured at 2 m and 2308 m are plotted in the figures. I will be reporting the values measured on this expedition in both metric and imperial, because the Youth Ambassadors who have joined this expedition are from different countries that use different scales of measurement for the same experiment. The youth ambassadors on this expedition are from Canada, Chile, and the United States of America.
Today, the expedition left San Predro de Atacama, Chile and traveled by bus, and then by jeep into Bolivia, traveling over two mountain passes in a time of 12 hours, almost all of it on gravel road, and sometimes on no road at all as the driver went cross-country over landscape that looked like the surface of Mars. In this region of the Andes Mountain range, there many volcanoes observable in all directions at all times. One volcano is currently active and we saw steam being released. In traveling over this mountain range, we crossed two passes that were at elevations of 4600 m (15090 ft) and 4900 m elevation (16,080 ft). We saw several small Salars, one which is the Salar de Blanco because of its salt, and adjacent to that was the Salar de Verde. The water of the Salar de Verde was a vibrant green because of the Copper Oxide that has eroded from the adjacent volcanoes and washed into the Salar. While at these altitudes, many of the expedition members noticed headaches, including myself (and lifting a duffle-bag that I know its all that heavy also caused me to lose my breath). Some members were sick, another fainted upon trying to get up from the table after lunch, while others didn't notice any effect at all. For the expedition members that experienced a headache, the cause was the low total pressure of air, and oxygen, at these elevation. Upon arriving in Uynui, Bolivia, after about 10 hours of travel all on gravel road. Uyuni is on the edge of the Salar de Uyuni which is at an elevation of ~3,500 m, my own headache had gone away. Tomorrow morning, the expedition members who enjoy coffee, like me, will be boiling water to make coffee. At that time, I'll be able to add another data point to the graphs plotting the boiling temperature of water versus elevation (and most importantly, I'll assess whether coffee made with water at 100 degrees centigrade tastes better than coffee made with water at ~90 degrees centrigrade).
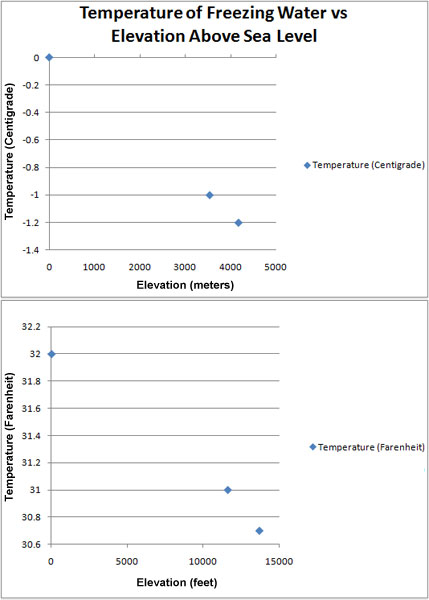
Update May 20, 2011: Bonus Freezing Water Data
School Results
Park Forest Middle School
Boiling point: 98.3° C
Elevation: 1330 feet (405 meters)
Pressure: 29.79 in Hg (1008.7 mb)
Dresden Elementary
Our elevations is 614 feet
The water boiled for us at 106°C
Coulee Kids
Our elevation in La Crosse, Wisconsin is 669 feet or 204 meters.
Our boiling point is 210°F or 99°C.
D'Arcy McGee/Symmes
Boiling: 100°C, 95°C, 100°C, 10°C0
Stabilized at: 106°C, 112°C, 115°C, 102°C respectively





























